Liquid Silicone Rubber (LSR) molding is a critical manufacturing process in the production of high-quality medical parts. Due to its biocompatibility, flexibility, and durability, LSR is widely used in medical applications such as seals, gaskets, diaphragms, and more. However, the presence of air traps during the molding process poses significant challenges. These defects can compromise the quality, functionality, and integrity of the molded parts, which is unacceptable in medical applications. This article examines the causes of air traps, their impact, and strategies to address them effectively.
Understanding Air Traps in LSR Molding
What Are Air Traps?
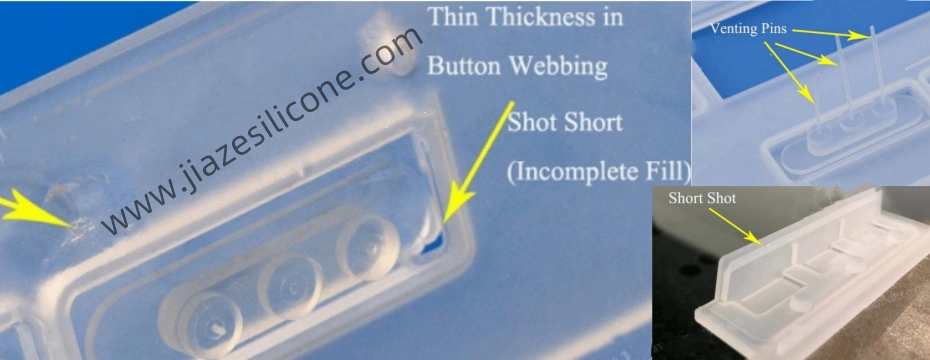
Air traps occur when air becomes trapped within the mold cavity during the injection molding process. These pockets of air can lead to surface imperfections, incomplete filling, and weakened areas within the molded part.

Causes of Air Traps
- Mold Design Issues: Poorly designed molds with inadequate venting systems are a primary cause of air traps. Complex geometries, sharp corners, or dead zones can restrict air flow and hinder proper venting.
- Improper Injection Parameters: High injection speed or pressure can cause the material to flow too quickly, trapping air in the process. Similarly, insufficient clamping force or low mold temperatures can exacerbate the problem.
- Material Properties: LSR’s low viscosity can contribute to rapid flow, increasing the likelihood of air entrapment if not managed correctly.
- Process Variations: Inconsistent process controls, such as fluctuating injection speeds or irregular heating profiles, can lead to air entrapment.
Impact of Air Traps on Medical Parts
Air traps are particularly concerning in medical applications due to strict regulatory and performance standards. The key impacts include:
- Structural Integrity: Trapped air creates voids that weaken the part’s structure, reducing its durability and reliability.
- Surface Defects: Visible air bubbles or blemishes compromise the aesthetic and functional quality of the part.
- Dimensional Accuracy: Air traps can result in incomplete filling of the mold, leading to dimensional inconsistencies.
- Regulatory Non-Compliance: Defective parts may fail to meet stringent medical industry regulations, potentially leading to recalls or legal consequences.
Strategies to Address Air Traps
Effective mitigation of air traps involves a combination of optimized mold design, precise process controls, and suitable material handling. Here are detailed strategies to address this issue:
1. Optimized Mold Design
- Improved Venting: Incorporate vents at strategic locations to allow trapped air to escape. Ensure vents are small enough to prevent material leakage but large enough for effective air evacuation.
- Balanced Flow Paths: Design the mold to promote even material flow, minimizing areas where air can become trapped. Use flow simulation tools during the design phase to predict and resolve potential issues.
- Avoid Dead Zones: Modify the mold to eliminate sharp corners or recessed areas where air can accumulate.
2. Injection Molding Process Optimization
- Controlled Injection Speed and Pressure: Gradually increasing injection speed and pressure helps to push air out of the mold cavity effectively.
- Vacuum-Assisted Molding: Implementing vacuum systems to remove air from the mold cavity before injection can significantly reduce air traps.
- Optimized Clamping Force: Ensure sufficient clamping force to prevent mold separation, which can introduce air into the cavity.
- Uniform Heating: Maintain consistent mold temperature to ensure smooth material flow and proper filling.
3. Material Handling and Preparation
- Deaeration of LSR: Degassing the material before injection reduces the likelihood of air bubbles being introduced into the mold.
- Proper Mixing: Ensure thorough and homogeneous mixing of the LSR components to prevent trapped air during material preparation.
4. Advanced Mold Technologies
- Micro-Venting Channels: Utilize micro-venting channels to enhance air evacuation without affecting part quality.
- Dynamic Mold Inserts: Incorporate movable inserts that adjust during the molding process to aid air evacuation in complex geometries.
5. Quality Control and Monitoring

- Real-Time Monitoring: Use sensors and cameras to monitor the molding process in real-time, allowing for immediate adjustments if air traps are detected.
- Regular Mold Maintenance: Periodically clean and inspect vents and flow paths to ensure they remain effective over time.
6. Simulation and Prototyping
- Flow Simulation Software: Use computer-aided engineering (CAE) tools to simulate material flow and identify potential air trap locations.
- Prototype Testing: Produce prototypes to validate the mold design and process parameters before full-scale production.
Case Study: Air Trap Resolution in Medical Diaphragms
A manufacturer of medical-grade diaphragms experienced issues with air traps, leading to high rejection rates. The following steps were implemented to resolve the problem:
- Redesigned the Mold: Added micro-venting channels and modified flow paths to ensure balanced material distribution.
- Vacuum Assistance: Integrated a vacuum system to evacuate air before injection.
- Process Adjustment: Optimized injection speed and reduced clamping force to allow for controlled material flow.
- Material Preparation: Implemented rigorous degassing procedures for the LSR material.
As a result, the rejection rate dropped by 85%, and the parts met all regulatory requirements.
Conclusion
Addressing air traps in LSR molding for medical parts requires a comprehensive approach encompassing mold design, process optimization, and material handling. Manufacturers can produce defect-free medical parts that meet stringent industry standards by adopting advanced technologies, maintaining rigorous quality control, and leveraging simulation tools. Ensuring the reliability and functionality of these components is paramount, as they often play a critical role in patient care and safety.